COVID-19 VACCINE UPDATES
Dear Patients,
I am sure that the news of COVID-19 vaccines is causing a mix of emotions, from fear of the unknown and hesitation about the side effects, to excitement about their potential to eventually return us to normal life. For that reason, I feel compelled to write this email. I hope this will give you an understanding of how the vaccines work, and why were they approved under EUA (emergency use authorization) by the FDA.
Please understand that there are various vaccine manufacturers working around the clock to accelerate the process of providing the vaccine to the high-risk population, healthcare workers and general public as soon as possible.
I will be discussing specifically the Pfizer-BionTech Covid-19 vaccine, which the FDA approved on Dec 11, 2020. The Moderna vaccine, still is in the approval process, shares the same technology as the Pfizer vaccine. Both use mRNA technology.
Let’s dig more into this: As we have learned, the spike protein, which is located on the virus surface, is the molecule that allows the Covid-19 virus to penetrate our cells (the host cells) and cause infection.
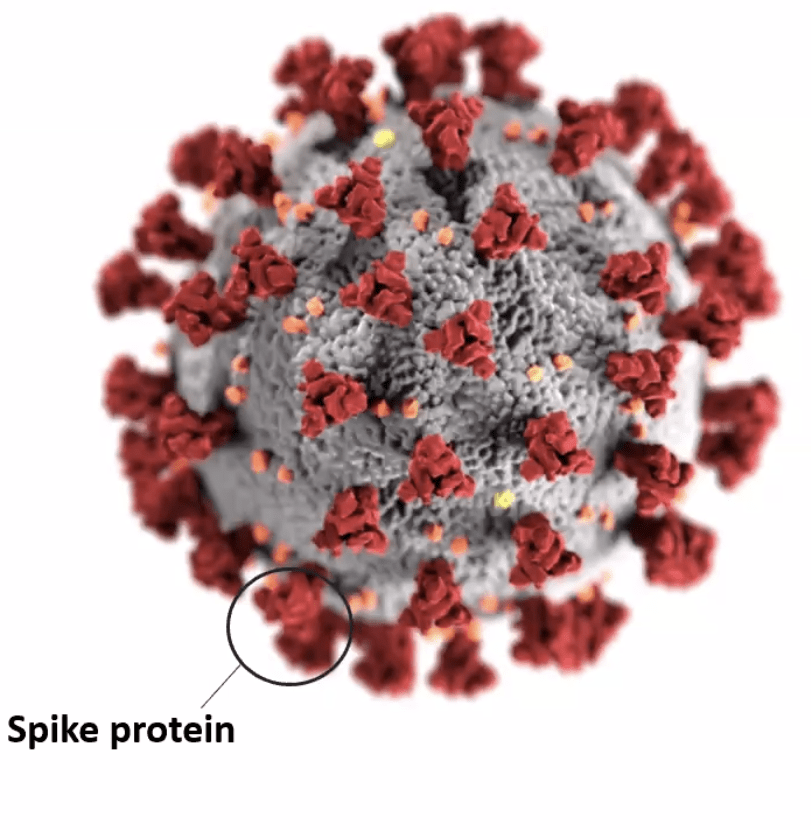
The Pfizer-BioNtech COVID 19 vaccine is a lipid nanoparticle-formulated mRNA vaccine that encodes or generates a protein in our cells. In this case it is the Covid-19 spike protein or viral spike (S) glycoprotein. The vaccine also has other ingredients which are stabilizers to prevent ingredient separation and sticking. The lipid nanoparticle protects the mRNA from degradation.
Here is the list of the ingredients of the COVID-19 vaccine:
Active Ingredients
- 30 mcg of a nucleoside-modified messenger RNA (modRNA) encoding the viral spike (S) glycoprotein of SARS-CoV-2.
- Fats
- lipids (0.43 mg (4-hydroxybutyl) azanediyl)bis(hexane-6,1-diyl)bis(2-hexyldecanoate), 0.05 mg 2[(polyethylene glycol)-2000]-N,N-ditetradecylacetamide, 0.09 mg 1,2-distearoyl-sn-glycero-3- phosphocholine, and 0.2 mg cholesterol)
- Salts
- 0.01 mg potassium chloride
- 0.01 mg monobasic potassium phosphate
- 0.36 mg sodium chloride
- 0.07 mg dibasic sodium phosphate dihydrate
- Sugar
- 6 mg sucrose
Please look at the diagram below, published in Nature on 04/30/2020 which focus on different vacciness models.
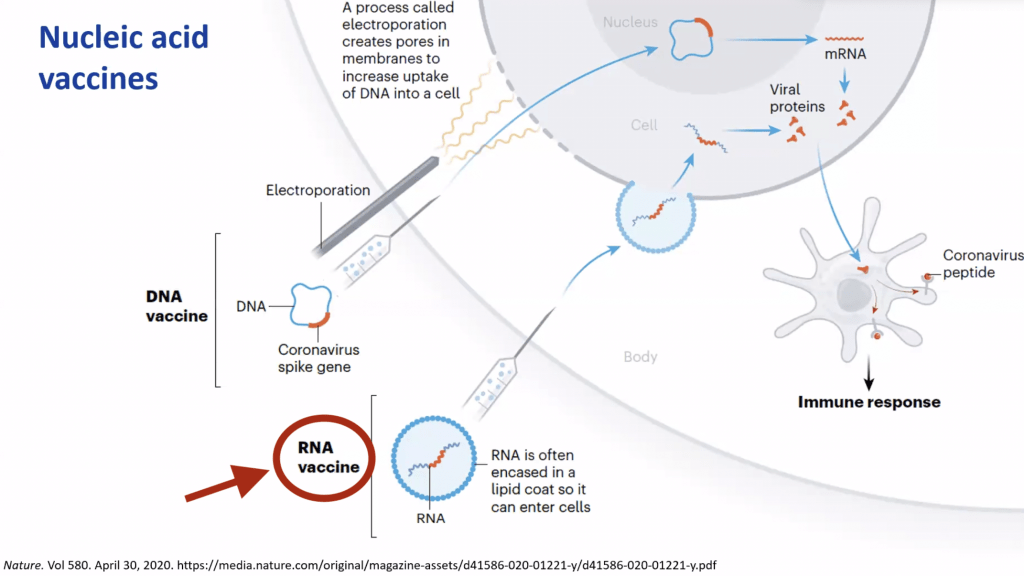
Let’s focus on the RNA vaccine area (red circle).
The vaccine contains messenger RNA that encodes for the spike protein of virus. Once injected into our muscle, the mRNA is released into the cytosol of the cell (not the nucleus- which is the one that contains the majority of our genetic material in form of DNA). This allows the cells to “create” the spike protein, which is the same as the virus. Once the spike protein is formed, our body recognizes it as foreign which, triggers the Antibodies formation and leads to T-cell activation to destroy the infected cells.
Please remember, the mRNA vaccine CAN NOT integrate into our own DNA or our own genome, nor will it change any genetic composition in our bodies.
This mRNA technology is new for vaccines, but it is NOT UNKNOWN. In fact, this technology has been studied for decades, mostly to encode cancer antigens (foreign substance that trigger an immune response in our body), to stimulate immune responses targeted at clearing or reducing malignant tumors.
Below is describing this technology in the past.
Examples:
- In 1990, researchers at the University of Wisconsin used this technology in mice.

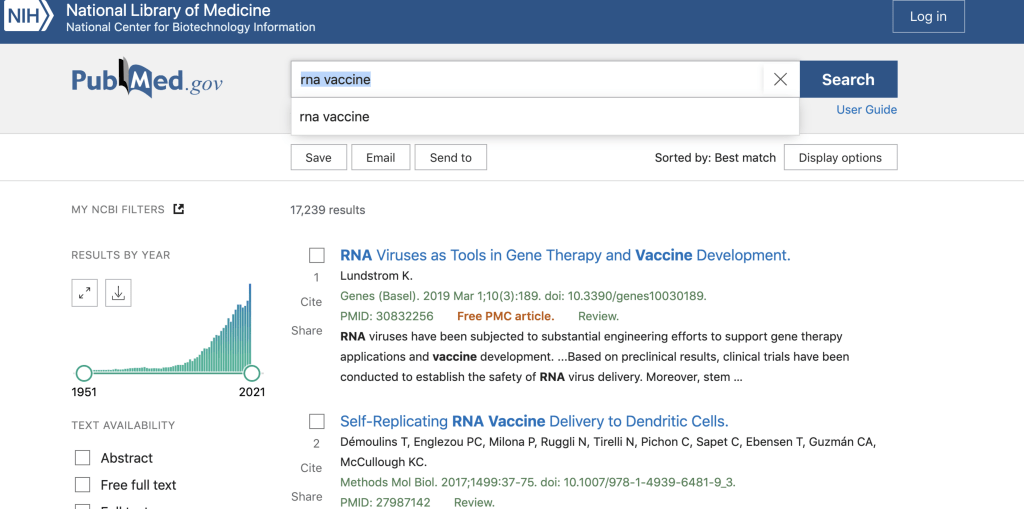
On the website pubmed.gov referencing RNA vaccines, there are 17,239 publications.
The RNA vaccines were also referenced in a January 2019 review discussing low-cost rapid vaccine manufacturing.

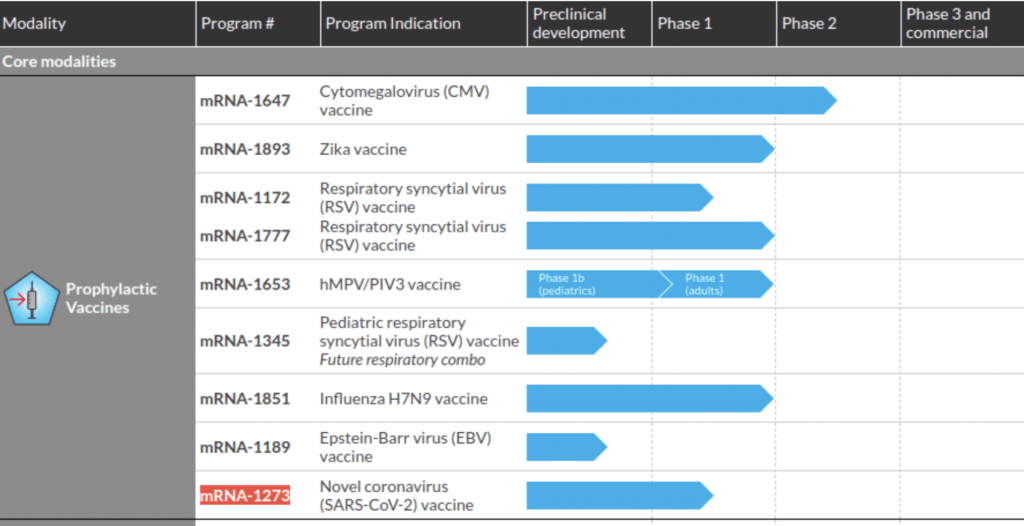
The Moderna graph below shows the company working on 8 vaccine developments, none in phase 3 (tested in humans). They are studying the mRNA vaccines for various viruses such Influenza, Zika, Rabies and Citomegalovirus (CMV).
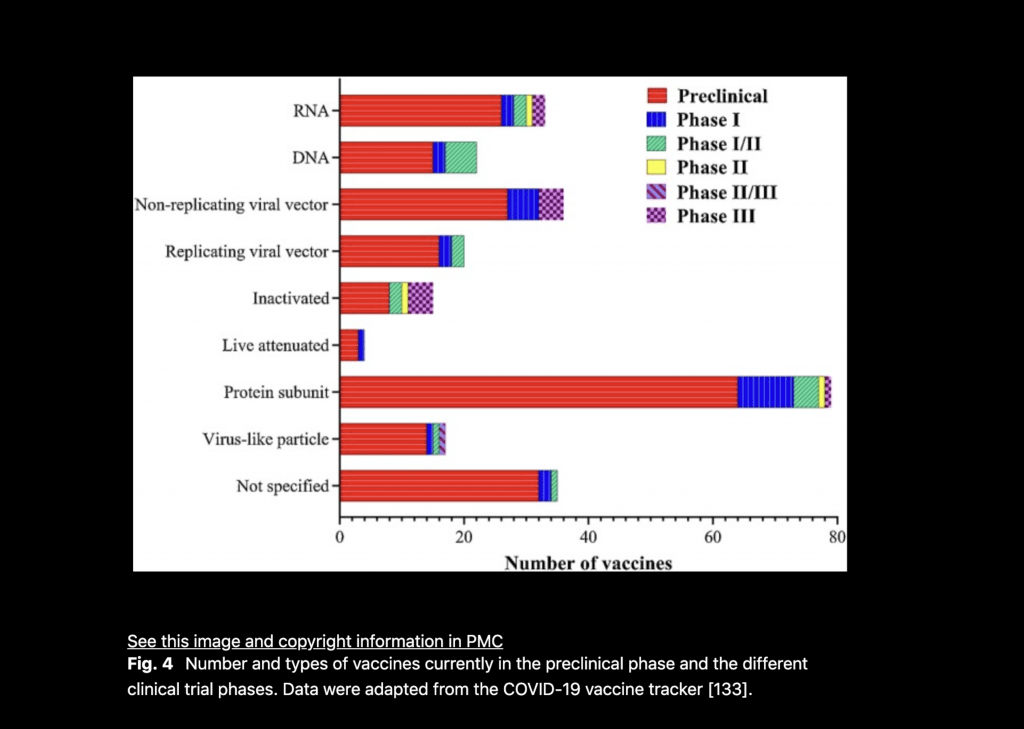
Below, are different ways the vaccines may work (RNA, DNA, no replicating vectors, Live attenuated virus, inactivated virus, virus like particle) and trials that are being conducted.
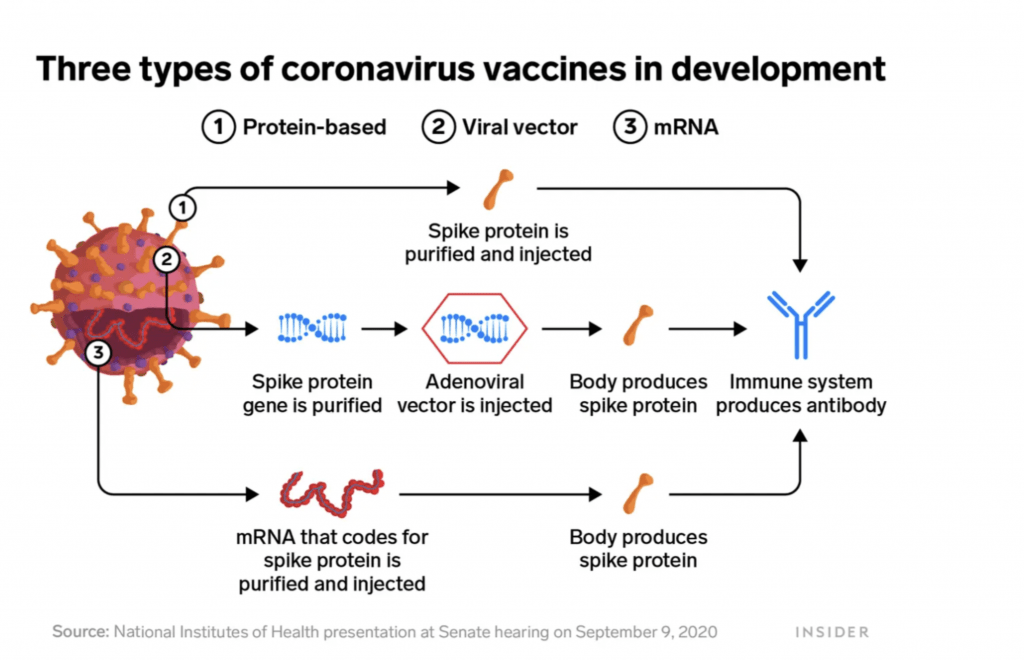
The graph below show the three types of coronavirus vaccines currently in development.
Below are specifics about vaccines. The information below was obtained from CDC website and virtual conferences I attended with the IDSA (Infectious Disease Society of America) over this past weekend.
- How is vaccine being administered?
- 2 doses intramuscular (IM) given within 3 weeks apart. If >21 days elapsed since first dose was given, dose needs to be administered at the earliest possible time.
- The Pfizer-BioNtech COVID-19 vaccine is not interchangeable with other COVID-19 vaccines. If your first vaccine was from Pfizer, then the second dose should be from Pfizer vaccine as well.
- You should not receive any other vaccines with the Pfizer one and you should wait at least 14 days before receiving any other vaccines. There is no data on safety and efficacy of receiving two vaccines at the same time.
- What are some of the most common short-term side effect reported?
- Vaccine recipients report local reactions (pain, erythema (redness), swelling, and systemic reactions such as fever, headaches, muscle aches.
- Reactions are more pronounced after the second dose.
- Most of the reactions were mild to moderate and resolved quickly.
- Note: the long-term side effects from the vaccine are not yet know.
- What are some important facts to know about receiving the vaccines?
- Vaccination should be offered to persons regardless of having history of prior COVID-19 infection, symptomatic or asymptomatic. There is no need to test for acute or prior COVID-19 infection prior to getting the vaccine.
- Patients with known Current SARS-COV-2 infection should wait 90 days prior to getting the vaccine. Re-infection is rare within 90 days.
- Patients who received passive antibody therapy for COVID-19 (monoclonal Antibody of Convalescent plasma), should wait 90 days prior to getting the vaccine.
- Patients with a known SARS-COV2 exposure should defer receiving the vaccine until they have completed their quarantine period. This will avoid exposure to others during vaccination visit.
- Patients in healthcare settings or long-term facilities may be vaccinated.
- The vaccine can be administered to persons with underlying medical conditions who have no contraindications to vaccination. Phase 2/3 clinical trials demonstrated similar safety and efficacy profiles in persons with underlying medical conditions, including those that are at increased risk for severe COVID-19 compared to persons without other comorbidities.
- Patients who are immunocompromised (example HIV or using immunosuppressive medications) and might be at increased risk for severe COVID-19. There is no data currently available to established safety and efficacy of vaccine in these groups. These individuals may still receive COVID-19 vaccine unless otherwise contraindicated.
- There is currently no data available on the safety of the vaccine for pregnant women. Animal studies are ongoing which are similar to human studies. Please remember the vaccines are not live and they do not enter the nucleus of the cell. Pregnant patients have an increased risk for severe illness (ICU admission, mechanical ventilation, and death) and increase in complications (preterm birth). If pregnant woman is part of a group (healthcare personnel) who is recommended to receive COVID-19 vaccine, she may choose to be vaccinated.
- There is no data on the safety of the vaccines for breastfeeding/lactating women. The mRNA vaccines are not thought to be a risk for breastfeeding infant, again consider if lactating woman is part of a group (healthcare personnel) who is recommended to receive a COVID-19 vaccine.
- The Pfizer vaccine efficacy with two doses is up to 95%.
- Routine prophylaxis to prevent symptoms after vaccine is administers is not recommended at this time. There is not enough information on impact of use on vaccine-induced antibody responses.
- Contraindications and precautions: There have been reports of anaphylactic reactions outside of clinical trials. It is recommended that in patients who have had a severe allergic reaction to any vaccine or injectable therapy (Intramuscular, or intravenous or subcutaneous) should NOT receive the Pfizer-BioNtech vaccine at this time. There have been two severe allergic reactions reported in UK. Patient with severe allergy were not included in the clinical trial.
A few final thoughts.
- Please remember that in order to get rid of the pandemic, we need to develop herd immunity (also called population immunity) which occurs when a population can be protected from a certain virus thereby, we reach certain levels of immunity with a vaccine.
- Remember, the more people who are vaccinated, the more rapidly we can get rid of this virus and pandemic and return to a sense of normalcy.
- In less than a year since the virus was sequenced, SCIENCE has developed vaccines and now, in only nine months, we have gained accessibility to vaccines.
- First. the vaccines will be available to front-line medical workers and population the older and more fragile population (age over 80 and nursing home residents).
- After the vaccine is given, there will be a tracker application, V-safe, which can be downloaded in a cell phone. It will be used as a tool to provide personalized health check- ins after you receive the vaccine.

